CAR T-Cell Therapy for Multiple Myeloma
CAR T-cell therapy (or chimeric antigen receptor therapy) is a relatively new type of treatment where cells are taken from your body and engineered to specifically recognize and attack multiple myeloma. This therapy can now be used for multiple myeloma that has not responded well to other treatments.
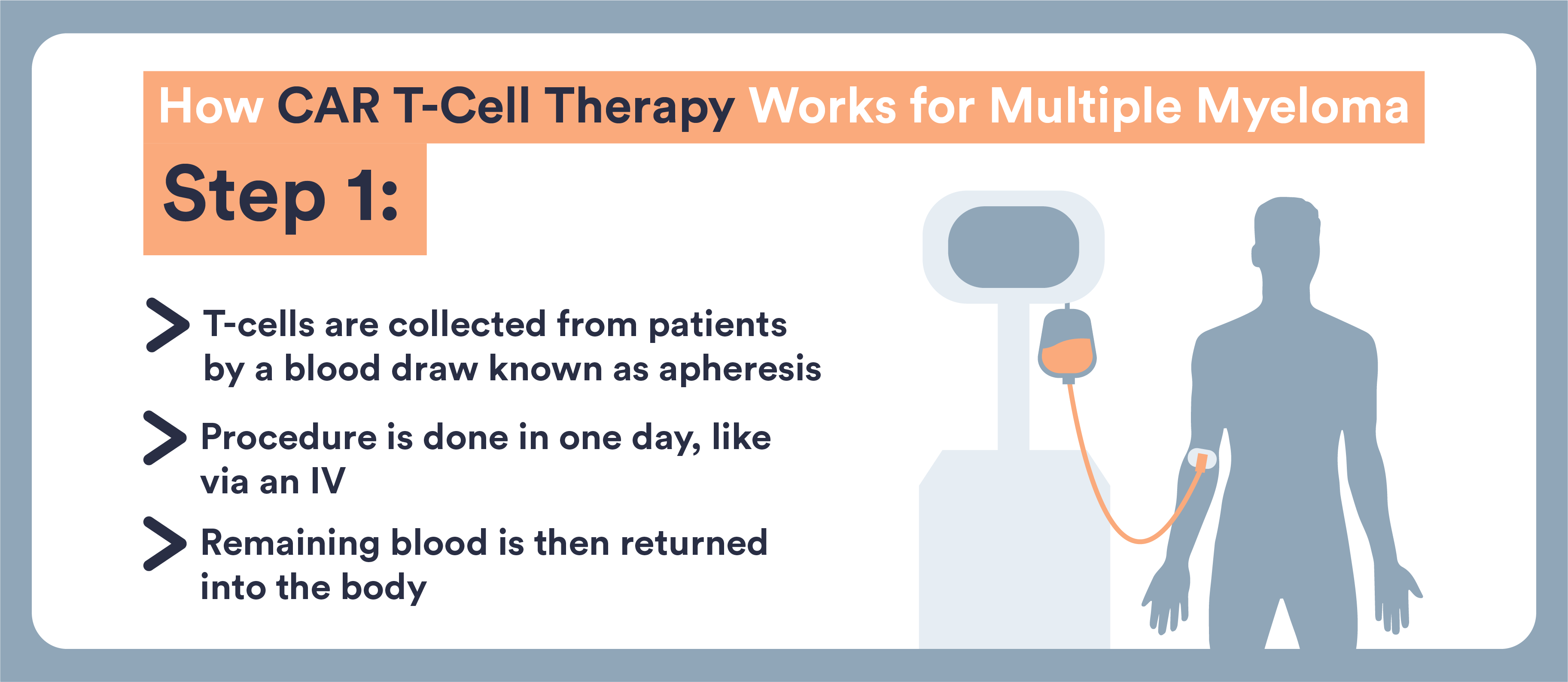
- CAR T-cells are immune cells that doctors remove from your body and re-engineer to target and destroy multiple myeloma
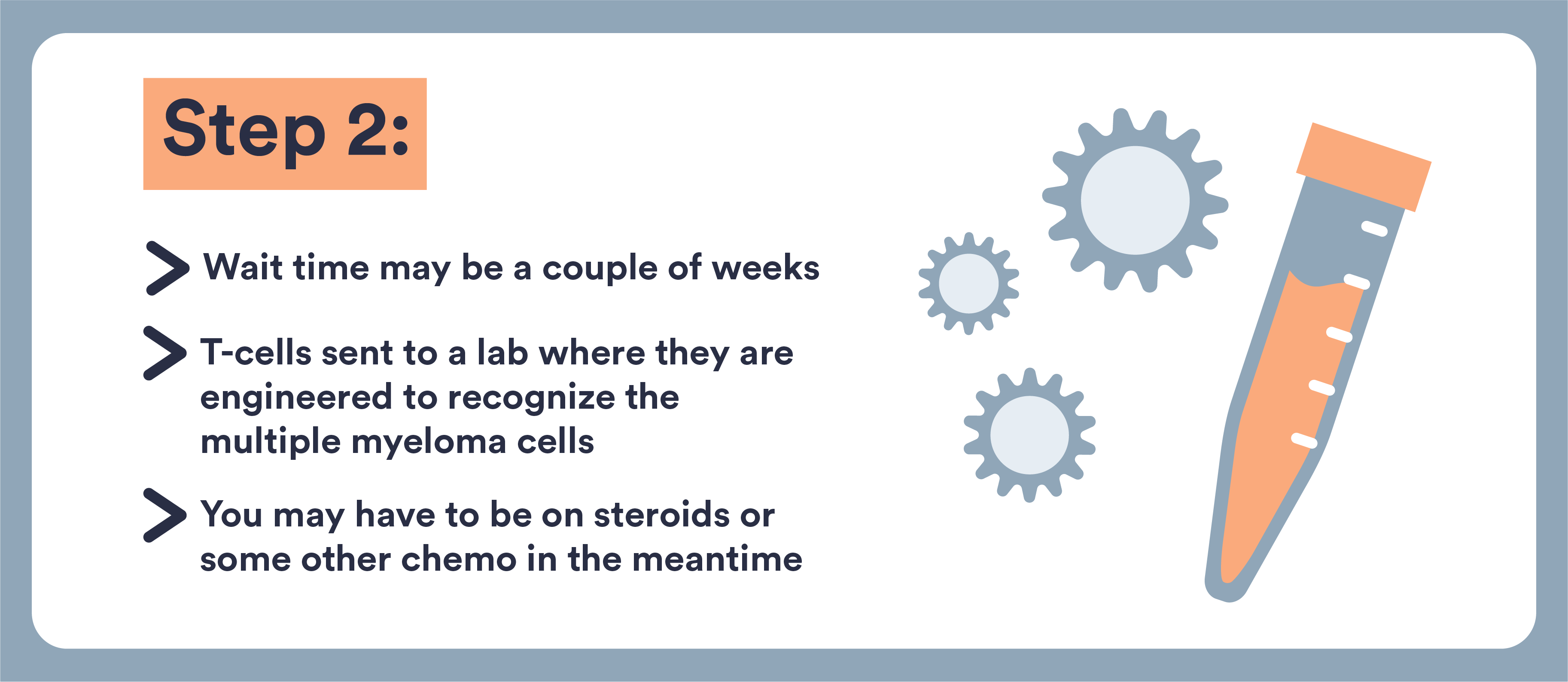
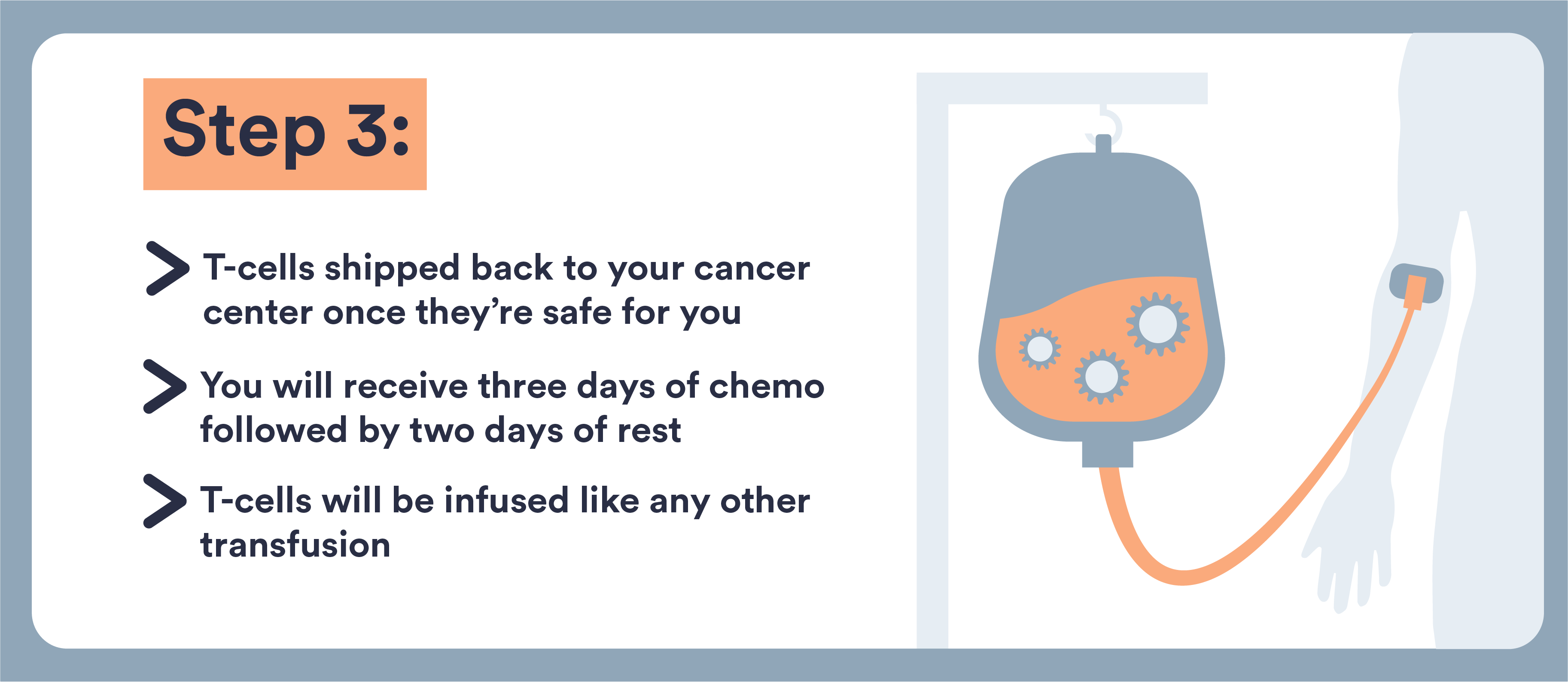
- These cells are removed from your blood, genetically altered, and returned to you
- This type of treatment can now be used for multiple myeloma that has not responded well to other treatments
CAR T-Cell Therapy - What You Need to Know
- CAR T-cells are immune cells that doctors remove from your body and re-engineer to target and destroy multiple myeloma
- These cells are removed from your blood, genetically altered, and returned to you
- This type of treatment can now be used for multiple myeloma that has not responded well to other treatments
- CAR T-cell therapy can cause side effects, some of which can be serious
- Cytokine release syndrome (or CRS) is the most common side effect and causes flu-like symptoms
- The brain can also be affected, which may lead to confusion
- Doctors closely monitor for side effects after treatment, and have therapies to manage them
Side Effects of CAR T-Cell Therapy
- CAR T-cell therapy can cause side effects, some of which can be serious
- Cytokine release syndrome (or CRS) is the most common side effect and causes flu-like symptoms
- The brain can also be affected, which may lead to confusion
- Doctors closely monitor for side effects after treatment, and have therapies to manage them
Safety Concerns Amid the Covid-19 Pandemic
Chimeric antigen receptor (CAR) T-cell therapy is a treatment that takes your own immune cells and re-engineers them to make them more efficient cancer fighters. It can be lifesaving for people whose cancer hasn't responded well to other therapies, or that has come back after treatment. But the process of harvesting yo... Read More
- More than 100 medical centers in the US offer CAR T-cell therapy for multiple myeloma and other blood cancers
- Currently, the treatment is approved for relapsed or refractory disease that has not responded well to at least four other treatments
- Clinical trials are ongoing to see if the treatment can be used earlier in the treatment process
The Treatment Plan
- More than 100 medical centers in the US offer CAR T-cell therapy for multiple myeloma and other blood cancers
- Currently, the treatment is approved for relapsed or refractory disease that has not responded well to at least four other treatments
- Clinical trials are ongoing to see if the treatment can be used earlier in the treatment process

Please confirm you are a US based health care provider:
Yes, I am a health care Provider No, I am not a health care providerSign Up Now.
Take Control of Your Disease Journey.
Sign up now for expert patient guides, personalized treatment options, and cutting-edge insights that can help you push for the best care plan.
