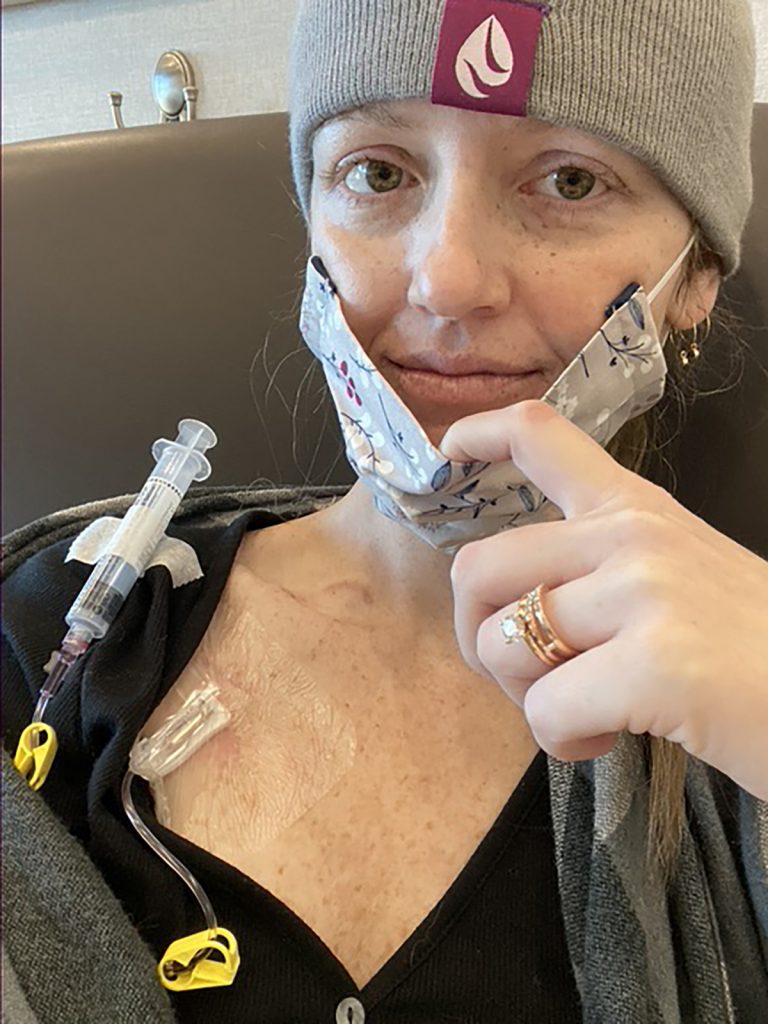
What is the Signatera Blood Test?
- FOX news anchor Lindy Thackston, 41, had been waiting weeks for the results of her second Signatera test, and the results are in: negative!
- The Signatera blood test is different from other cancer-detecting blood tests in that it can detect if cancer is still present in patients who have previously been diagnosed with cancer patients like Thackston.
- While the test is not yet approved by the FDA (and therefore not part of the National Comprehensive Cancer Network guidelines), doctors are able to use it in their practice.
- Signatera is typically ordered every three months, reducing or eliminating the necessity to order frequent scans. The first Signatera test should be taken three to four weeks after surgery.
The Indianapolis-based television news anchor had been waiting weeks for the results of her second Signatera cancer blood test, and the results are in: negative!
Read MoreView this post on Instagram
In a previous interview with SurvivorNet, Thackston said she took the first Signatera test on Nov. 1 of last year, and it took more than two months to get the results.
"Then, (on) January 3, which is actually my wedding anniversary, we were driving to go out to eat and … my oncologist called and said it was negative," she said, "and we were shocked because that was not expected at all."
Shortly after receiving the results of her first test, she took a second test, but was informed that her results were lost in the mail. She had to take a third test, which is the test she just received the results for.
What is the Signatera Blood Test?
Catching cancer early is the key when it comes to treatment, and there are a few different blood tests available that claim to detect cancer in its early stages.
However, the Signatera blood test is different in that it can detect if cancer is still present in patients who have previously been diagnosed with cancer patients like Lindy Thackston.
The Signatera test can be used to detect minimal, or molecular, residual disease (MRD) following surgery, to monitor a patient's response to cancer treatment or detect cancer recurrence following a period of remission, according to test-maker Natera, Inc., a clinical genetic testing company based in Austin, Texas. (There are competing tests, such as ones from Guardant Health, in this space in addition to Signatera. Guardant Health has commercially launched liquid biopsy-based Guardant360®, Guardant360 CDx and GuardantOMNI® tests for advanced-stage cancer patients, and Guardant Revealâ„¢ for early-stage cancer patients.)
Speaking with SurvivorNet about the Signatera test, Dr. Adham Jurdi, medical director for oncology at Natera, explained that the test is used by doctors after surgery to make sure they got all the cancer during the procedure. This also allows for pieces of the patient's tumor to be used to customize the test, essentially creating an individualized map of how the patient's body makes the tumor.
If the test comes back negative, like in Thackston's case, it spares the patient from going through another round of unnecessary and harmful treatment, adding that it is a "huge quality of life issue."
While the test is not yet approved by the U.S. Food and Drug Administration (and therefore not part of the National Comprehensive Cancer Network guidelines), doctors are able to use it in their practice. One of those doctors was Dr. Adham. In fact, he loved the Signatera test so much that he wanted to work for the company that made it. He was previously an oncologist and medical director at Austin Cancer Center in Austin, Texas, but joined the Natera team in November of last year.
Signatera is typically ordered every three months, generally along with relevant serum protein biomarkers, according to Natera. This reduces or eliminates the necessity to order frequent scans for patients. The first Signatera test should be taken three to four weeks after surgery.
"It (Signatera) is the next big thing in cancer care," Dr. Adham said. "It is basically moving the way we monitor and treat cancer patients … into the 21st century."
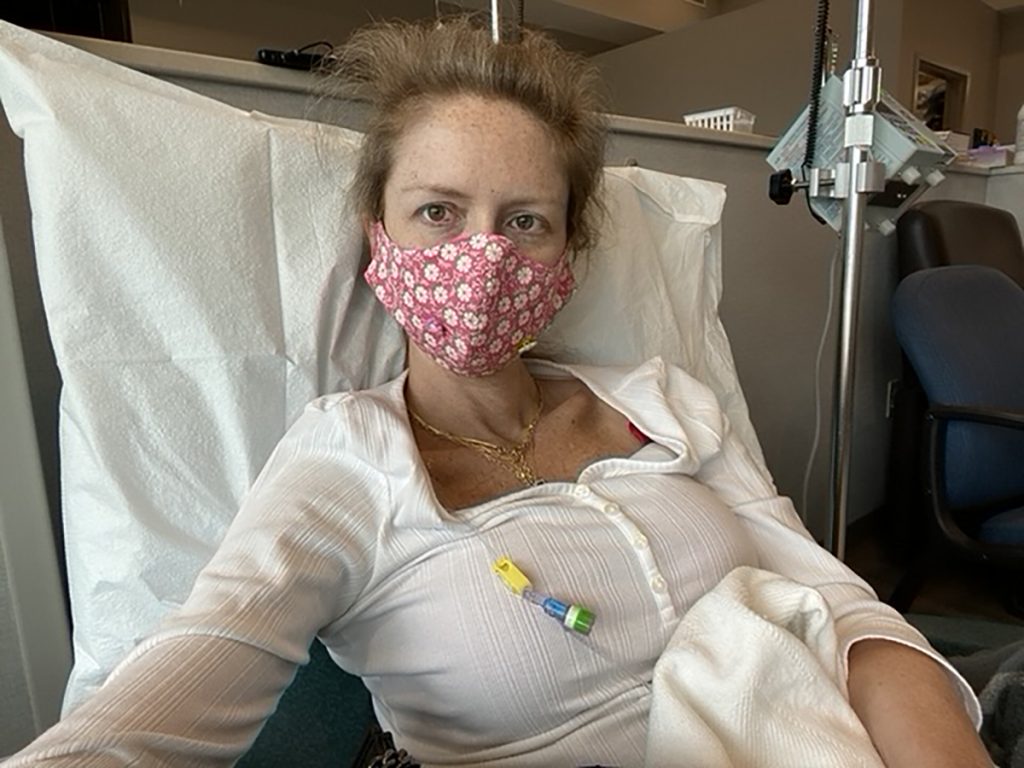
Lindy Thackston's Cancer Battle
Lindy Thackston, a beloved television news anchor for FOX59 News in Indianapolis, was diagnosed with stage 3 colorectal cancer in May 2020. In the spring of 2021, she finally finished all 10 rounds of chemotherapy to fight off her cancer.
But in September of that year, she shared that doctors found a spot on her lung during an unrelated hospital visit. She told her thousands of Instagram followers that the lung spot prompted a biopsy, which revealed the cancer had spread to her lung her cancer had reached stage 4.
In an episode of her podcast, Life With Lindy, posted that same month, she further explained that her doctors think the spot on her lung had been there from the very beginning when she was diagnosed with stage 3 colorectal cancer.
Management of Metastatic Colon Cancer
Thackston disclosed to SurvivorNet that this discovery also meant she was actually stage 4 when she was diagnosed in May 2020, considering the cancer had spread beyond its origin organ. But the spot was small; it never changed or did anything the entire time she battled colorectal cancer, she said.
"That's actually good news because it means a new spot didn't pop up," she told SurvivorNet.
Dr. Heather Yeo, a surgical oncologist and colorectal surgeon at New York-Presbyterian/Weill Cornell Medical Center, previously told SurvivorNet that when colon cancer spreads outside the colon, "most commonly spreads to the lung and to the liver." In Thackston's case, it had spread to her lung.
Thackston ended up having a lobectomy procedure (surgery where an entire lobe of the lung is removed) to remove half of her left lung.
"Then the question was, 'OK, do I do a round of chemo after that, or not?'" Thackston said.
Thackston consulted with her oncologist at St. Vincent Hospital (in Indianapolis) about what her next steps should be chemotherapy, or not. She also sought second opinions on what to do at Indiana University Health and the Mayo Clinic.

"I had IU and Mayo (Clinic) telling me that they would not do chemo and that they would just continue to constantly monitor me," Thackston told SurvivorNet. "My oncologist, who I've been going to for the whole time, was leaning toward chemo. And I obviously didn't want to do it. … We ultimately decided not to do it."
"It was just a personal decision," she added, "and so (at that point), it was time to take another Signatera test again."
"Hopefully it's negative again," she added. "If it's positive, then I'm gonna have to figure out where the cancer is and start over."
Luckily for Thackston, the test result was negative, so she doesn't have to start from square one again. However, she'll now take yet another Signatera test in April, as well as begin getting PET scans every three months to monitor her remission progress.
From everyone here at SurvivorNet, congratulations, Lindy!
Learn more about SurvivorNet's rigorous medical review process.


