Key Takeaways for Atlanta Patients
- CAR T-Cell Therapy offers new hope for people with relapsed or refractory large B-cell lymphoma, and it’s available at top Atlanta hospitals like City of Hope.
- The treatment involves several steps: evaluation, T-cell collection, cell manufacturing, short chemotherapy, and infusion. Followed by careful monitoring for side effects.
- Common side effects, including temporary fever or confusion, are generally short-lived and managed by experienced medical teams.
- Most patients stay in the hospital for about a week, then continue regular follow-ups nearby with strong caregiver and family support.
Doctors are learning that with diffuse large B-cell lymphoma, waiting too long can give the cancer time to grow and become harder to treat. Recent studies show that CAR T-Cell Therapy could be an option much earlier, sometimes right after the first chemotherapy doesn’t succeed.
Where Do I Begin?
Read More- Have you had the required prior treatments (typically at least one or two lines of chemo-immunotherapy) and did the lymphoma relapse or become refractory?
- Is your overall health (heart, lungs, kidney, liver function) good enough to tolerate this treatment?
- Is the designated cancer center certified and experienced in CAR T-Cell Therapy?
- What are the logistics and support services (accommodation, monitoring, caregiver responsibilities) for the time you’ll spend in the hospital/local area?
“We start talking about CAR T-Cell Therapy immediately. The sooner we can start working with a patient with relapse the better because there is a preparation period, because I would say it may take a week or two for insurance approval,” Dr Leslie Popplewell, Medical Director of Hematology and Blood and Marrow Transplant at City of Hope Cancer Center Atlanta, told SurvivorNet.
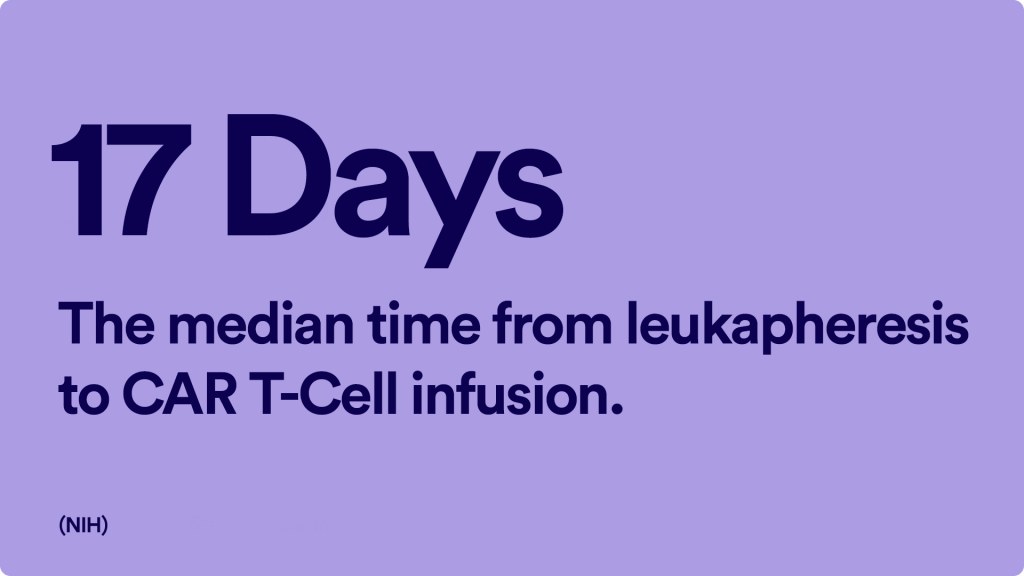
After Insurance Approval: Leukapheresis
“Once that approval is obtained, then we can set up the patient for the collection of the T-cell. That collection can take place pretty quickly once we get authorization. It doesn’t require a lot of preparation time. We can collect all the T cells we need in one session, which would be one morning or one afternoon,” Dr. Popplewell added.
The T-cell collection is also known as Leukapheresis. Here’s how it typically works:
- You arrive at the treatment center (often outpatient or day-hospital) and have two IV lines (or sometimes a central line) placed: one draws your blood, the other returns it after cell separation.
- Your blood passes through a machine that separates out T cells and returns the remainder to you. This may take 2-4 hours.
- You may feel mild discomfort: tingling, muscle spasms (due to low calcium during the procedure), or light fatigue. These are manageable, and the care team will monitor you.
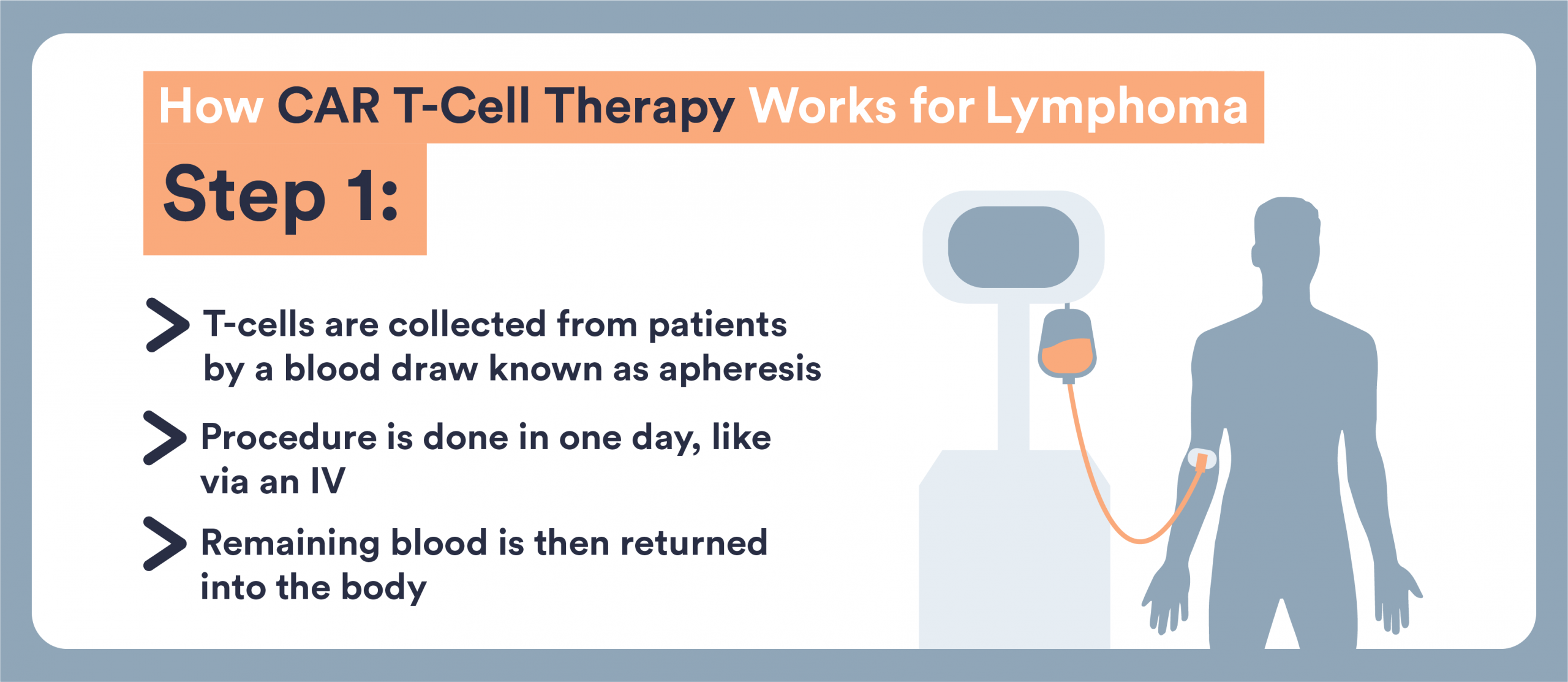
Manufacturing Period
After collection, your T cells are sent to a specialized laboratory where they will be genetically engineered to become CAR T-Cells.
“After that, it may take about two weeks [for the treatment to be ready]. The manufacturer of those CAR T-Cells, they have to go away to a manufacturing facility and during that period of time patients may or may not need some additional treatment,” explains Dr. Popplewell.
While your engineered T cells are being made, you’ll continue to be monitored. At some point, you might receive a short course of lymphodepleting chemotherapy (sometimes called preparative chemo) just ahead of the CAR T-Cell infusion.
This chemo serves two key purposes:
- It reduces some of your existing immune cells so the newly-engineered CAR T-Cells will have “space” to expand and act.
- It lowers interference from other immune responses, giving CAR T-Cells a better chance to grow and kill lymphoma cells.
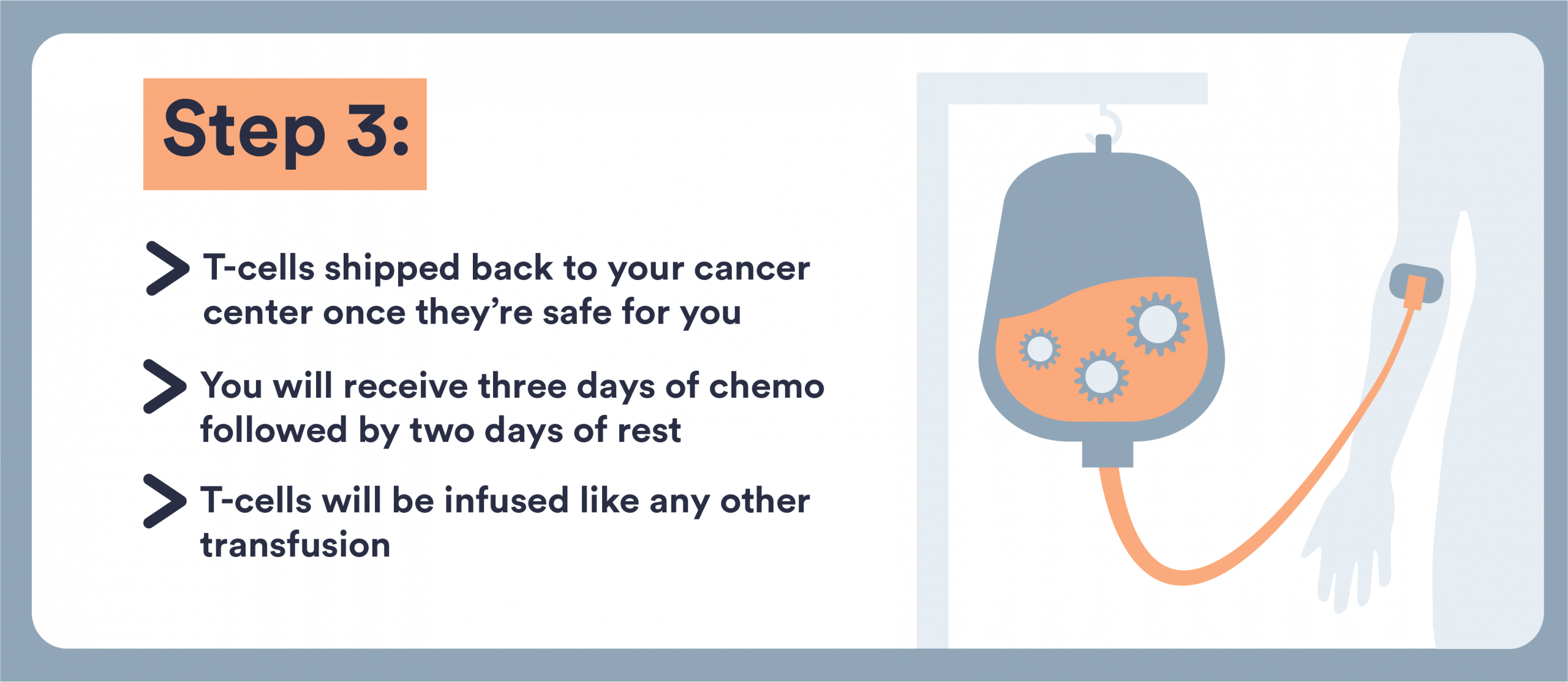
CAR T-Cell Infusion
This is the day the engineered cells are returned to your body. Think of it as a “living drug” being infused back.
“Once the manufacturer is complete and the cells have gone through their quality testing. We know they’re ready to go. Those cells can be shipped to our hospital and then the patient will be admitted,” Dr. Popplewell says.
Here’s what to know:
- The infusion is done in a hospital certified to administer CAR T-Cell Therapy
- You’ll be admitted as an inpatient or monitored as an outpatient (depending on center, risk and which product is used).
- The actual infusion may take a short time, but you’ll remain under watch for immediate reactions (fever, chills, low blood pressure, allergic reactions)
- After infusion, the CAR T-Cells begin to multiply and do their job: identify and attack lymphoma cells that express the target antigen
- After CAR T-Cell Therapy, it’s common for the immune system to be weaker for a while because the treatment not only targets cancer cells, but can also temporarily affect normal immune cells that protect you. As a result, patients are at higher risk for infections especially in the first few months after treatment. These infections can include common ones like colds or pneumonia, as well as more serious viral or bacterial infections. Doctors carefully monitor patients for signs of infection, and you may receive antibiotics, antivirals, or other medicines to help prevent or treat them
What About Side Effects
Once the CAR T-Cells are infused into someone’s body, they multiply over the first week. While the CAR T-Cells are killing cancer cells, they also produce inflammation and a collection of symptoms known as cytokine release syndrome.
One of the main symptoms is a fever, which can spike as high as 104 degrees Fahrenheit. Some people will develop more serious symptoms such as low blood pressure, difficulty breathing, or damage to organs such as their heart, lungs, kidneys, or liver.
Cytokine Release Syndrome (CRS)
As CAR T-cells kill cancer, they release cytokines: chemical signals that call other immune cells into battle. When this response becomes too strong, the body experiences systemic inflammation, which can cause fever, low blood pressure, and breathing problems. In short: CRS happens because the immune system becomes hyper-activated.
The good news is, cytokine release syndrome is usually short-lived, Clinical trials of commercially available CAR T-Cell Therapies showed a median time of CRS onset of 2 to 3 days with a median duration of 7 to 8 days.
The rate of severe cytokine release syndrome is only around 7%.
Brain Fog
“There is another side effect of CAR T-Cells called neurotoxicity or ICANS. Where patients can develop some confusion,” Dr. Popplewell explains.
ICANS stands for Immune effector cell-associated neurotoxicity syndrome. This includes confusion, difficulty speaking, tremors, seizures/convulsions in rare cases.
“For this reason, patients really need to be closely monitored. They need to have a caregiver available 24 hours a day in those days after the CAR T-Cell infusion,” adds Dr. Popplewell.
A Brief Summary
CAR T-Cell Therapy involves several key steps spread out over a few weeks:
- Consultation and Evaluation: You’ll meet with the CAR T-Cell Therapy team (oncologists, nurses, and specialists) to review your medical history and confirm eligibility.
- T-Cell Collection (Leukapheresis): Your T-Cells are collected through a machine that filters your blood.
Manufacturing Period – Your cells are sent to a lab for engineering. This step can take 2–4 weeks, depending on the product. - Bridging Therapy (if needed): If your cancer is growing quickly, your doctor may give temporary chemotherapy while waiting for your CAR T-Cells to be ready.
- Lymphodepleting Chemotherapy: A short, low-dose chemotherapy regimen (usually 3 days) helps prepare your immune system.
- CAR T-Cell Infusion: The modified cells are given back through an IV, usually in the hospital. The infusion itself takes less than an hour, but you’ll be monitored closely afterward.
After infusion, you’ll stay in the hospital for at least 1 to 2 weeks so your care team can watch for early side effects and manage them promptly. You’ll then continue to have frequent follow-ups for several weeks or months.
Learn more about SurvivorNet's rigorous medical review process.